
Systemic arteries are mainly pressure vessels. Their walls contain a higher proportion of elastic and muscular tissue than veins. They are vessels with less capacity to distend than veins . Veins on the contrary are mainly capacitance vessels and can distend more in response to an increase in blood volume. Since both arteries and veins are interconnected by the capillaries and the whole closed system is filled with a volume of blood, a greater fraction of the total blood volume will accommodate in the veins that can distend more easily. The values of blood volume distribution given above are for a fully functional system in which the heart is pumping. What do you think would happen if the heart stopped?


Although we could say that among the those described above the transport of essential substances to the tissues may be the most significant function of the circulatory system , as you can see all of the other factors are of paramount importance in the maintenance of internal homeostasis. Each function depends on the other. For example, if the subcutaneous and muscle circulation does not increase during strenuous exercise, the body is not capable of releasing the heat produced by the higher metabolism. The result is hyperthermia with eventual heat shock and death.
Our circulatory system is a closed circuit containing a pump (the heart) that serves to maintain a pressure gradient sufficient to sustain an effective blood flow from the distributing ducts or arteries to the thin vessels or capillaries and back through the collecting ducts or veins. Two circuits, the pulmonary and systemic are present. The pulmonary may be considered the low pressure circuit while the systemic is the high pressure one.

The pressure in our systemic circulation drops gradually from an initial 120mm Hg in the aorta to a minimum of 2mm Hg in the Vena Cava. The main drop in pressure occurs in the resistance vessels or arterioles which are highly muscular and well regulated by nervous and humoral factors. They are also muscular arteries containing a high proportion of vascular smooth muscle in the vessel walls. Compare them to the aorta and veins in terms of elastic tissue/smooth muscle content. At 2mm Hg in the inferior vena cava, what factors do you think propel blood into the right heart from the lower parts of the body?
electrocardiac physiology
Yes, you must remember these equations always. The first two are Nernst equations for equilibrium potentials of potassium and sodium respectively. The last one is the Goldman equation that takes into account the effect of other ions and their permeability coefficients (P). If you consider that the permeability of potassium is 100 times higher than that of sodium then the effect of sodium and chloride becomes negligible in the Goldman equation and for all practical purposes it becomes the Nernst equation for potassium.
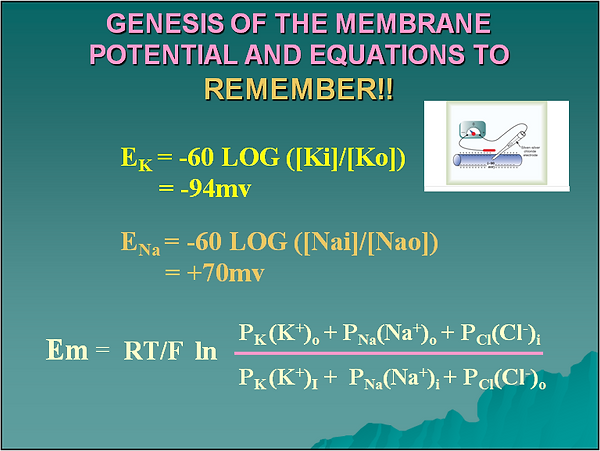

Like in many other cells the resting membrane potential of the cardiomyocite depends mainly on the diffusion of potassium from the intracellular to the extracellular compartment. If you consider that, for practical purposes only, the cell membrane is permeable mainly to potassium, then ,as potassium diffuses out of the cell following its concentration gradient, positive charges move out of the cell not accompanied by negative charges. This creates a very small region of positive charge in the outer part of the cell membrane and consequently another very small region of negative charge inside. The negative charge inside tends to keep the K+ inside the cell and works against the chemical force created by the concentration gradient that tends to move K+ out of the cell. When both forces are equal net K+ movement across the membrane is zero and a potential difference has been established across the cell membrane.
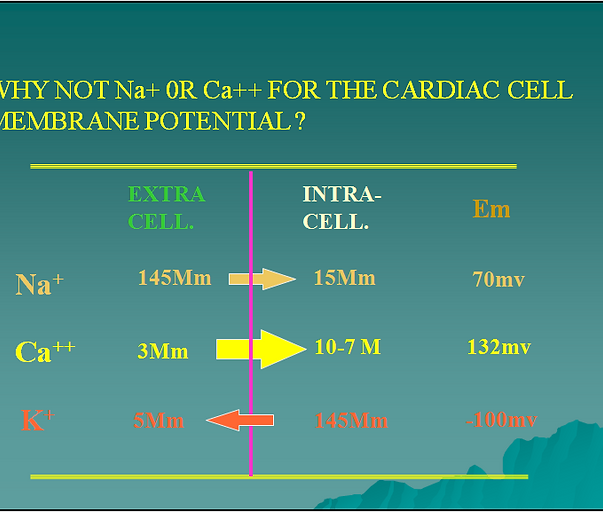
If you use the normal intra and extracellular concentrations of Na+ or Ca++ to calculate the resting membrane potential of a myocardial cell the values obtained deviate significantly from the experimentally measured values. Thus, the distribution of these ions across the cell membrane does not explain the measured resting membrane potential. When the extra and intracellular concentrations of K+ are used in the Nernst equation the value obtained for Em approximates the measured value indicating that K+ is a major factor in the resting membrane potential. In addition when the permeability of K+ and Na+ are compared, K+ shows up as 100 times more permeable than Na+. K+ becomes a very important and CLINICALLY RELEVANT ion in cardiac function. More later.


Microelectrodes placed in individual cells of the atria, ventricles and sinoatrial node show distinct action potential profiles. You should recognize these profiles at first sight and without hesitation. Between the atrial and ventricular action potential profiles the main differences lie in the plateau phase that is more pronounced in the ventricular cell. The nodal cell action potential is easy to recognize since there is no plateau phase, the depolarization phase is slower than in the atrial or ventricular cell and the resting membrane potential is achieved briefly as the cell continues to depolarize automatically until the next action potential is triggered
Both atrial and ventricular fibers are fast response types. The graph shows an electrophysiological recording obtained from a single ventricular fiber. To obtain the recording a microelectrode is placed in the intacellular compartment of the cell and a second microelectrode is left outside as a reference. The very small signal is then amplified (AMP) and sent to the horizontal plates of an oscilloscope. As the cell depolarizes and repolarizes in each action potential we can measure the voltage in the vertical deflection of the oscilloscope electron beam. The horizontal deflection of the beam gives us the time. For the ventricular cell the action potential has been divided into 4 phases. Phase 0 is the rapid depolarization, phase 1 is a transient repolarization, phase 2 is the plateau phase, phase 3 is the repolarization phase and phase 4 is the resting potential phase.


Phase 0 is the depolarization phase of the fast fiber action potential and it’s mostly dependent on the opening of fast Na+ channels in the cell membrane controlled by m (activation gates) and h (inactivation gates). At the resting membrane potential (A) the activation gates m close the channel maintaining Na+ out of the cell. The electrical and chemical gradient for Na+ favor its entry into the cell (arrows). When the cell depolarizes and the membrane potential drops to near -65mv (B), the m gate moves out of the channel leaving an opening through which Na+ can move into the cell. As Na+ moves into the cell the electrical gradient that promoted its entry dissipates and then reverses (C to E). The chemical gradient continues to promote Na+ entry (black arrow). After reaching about +30mv the h gate closes or inactivates the channel ending the rapid depolarization phase or phase 0. As the cell repolarizes the gates are returned to their normal resting state (A). This is the tetrodotoxin sensitive phase of the cardiac cell action potential
Three K+ channels are involved in the repolarization period of the fast fiber action potential. The small repolarization that occurs in phase one depends on the Ito channels. This channels are also important in the final repolarization period and in fact if you observe an atrial cell action potential, the Ito effect will be more pronounced thus shortening the plateau period or phase 2. In phase 2 the K+ efflux through the Ito,Ik and Ikl channels cannot counteract the Ca++ influx and the potential remains unchanged. Later on the three K+ currents counteract the Ca++ influx and repolarize the cell in Phase 3. It is in this phase that the Ik channels have a significant effect. As the membrane potential increases during repolarization from -20 to -70mv the Ikl channels acquire more significance and become a major factor in repolarization and eventual maintenance of the resting membrane potential

L and T type calcium channels open during phase 0 of the fast fiber action potential. The L channels or “Long Lasting” open rapidly but deactivate slowly. These are the most abundant of the Ca++ channels. The T channels or “Transient channels” open before the L type but close much more rapidly. The opening of the Ca++ channels brings a huge influx of Ca++ into the cell that coupled to the efflux of K+ maintain the membrane potential almost unchanged for a period of time. This is the cause of the plateau phase of the fast fiber action potential. Eventually the Ca++ channels close and K+ efflux predominates bringing about the eventual repolarization. Please note that the L Type Ca++ channels are the ones in which the calcium blockers have their inhibitory effect. Remember also that the Ca++ that enters the fast fiber during Phase 2 is actively used for contraction
