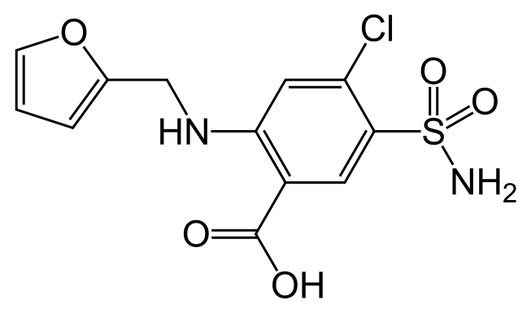



Thiazides achieve their diuretic action via inhibition of the Na+/Cl− cotransporter (NCC) in the renal distal convoluted tubule . The NCC facilitates the absorption of sodium from the distal tubules back to the interstitium and accounts for approximately 7% of total sodium reabsorption . By decreasing sodium reabsorption, thiazide use acutely results in an increase in fluid loss to urine, which leads to decreased extracellular fluid (ECF) and plasma volume. This volume loss results in diminished venous return, increased renin release, reduced cardiac output and decreased blood pressure .
Note
Within days, the reduction in cardiac output increases total peripheral resistance (TPR), which stems mostly from activation of the sympathetic nervous system (SNS) and renin–angiotensin–aldosterone system (RAAS) . This acute effect is evidenced by the fact that an infusion of dextran, a volume expander, during the acute thiazide treatment phase restores blood pressure to pretreatment levels .
Chronically, however, thiazides must lower blood pressure via some other mechanism. Plasma and ECF volumes almost fully recover within 4–6 weeks of thiazide initiation, yet blood pressure reduction is maintained . After chronic administration, discontinuation of thiazides result in a decrease in renin levels and rapid volume replenishment, although the rise in blood pressure is much slower ]. In addition, when given a dextran infusion, patients taking thiazides over longer periods of time (>2 months) experience an expansion of body fluid volume but blood pressure does not increase to baseline . In addition, if diuretics simply lowered blood pressure by reducing plasma volume, then one would expect loop diuretics (such as furosemide, torsemide and bumetanide), which are superior diuretics to thiazides, to be superior antihypertensives. However, loop diuretics do not lower blood pressure to the degree thiazides do (although some difficulty exists in establishing equivalent doses) . The aforementioned evidence indicates that the chronic thiazide antihypertensive effect is not exclusively due to a loss of blood volume.Research has shown for decades that a decrease in TPR plays an important role in the antihypertensive effect of thiazide diuretics over the long term . However, the mechanism of this decrease in TPR has not been fully elucidated. Many hypotheses exist to describe this mechanism
Many have hypothesized that the thiazide-associated decrease in TPR could be the result of a direct vasodilatory effect, perhaps separate from the diuretic effect . Multiple reports suggest a reduced vasoconstrictive effect of various pressor agents in animals and humans pretreated with thiazides . However, these findings do not specify whether the effect was by direct vasodilation or stemming from an indirect action, such as volume depletion
Direct action on the endothelium has been implicated in the acute vascular actions of thiazides. Methaclothiazide inhibited norepinephrine-induced vasoconstriction in the aortas of spontaneously hypertensive rats, but not in Wistar–Kyoto (non-hypertensive) rats . Removal of the endothelium abolished this response in spontaneously hypertensive rat aortas, as did treatment with Nω-nitro-L-arginine, a nitric oxide synthesis inhibitor
In addition, HCTZ and indapamide were found to dilate guinea pig mesentery arteries in vitro [. When arteries were cotreated with charybdotoxin, an inhibitor of the large conductance calcium-activated potassium (KCA) channel, HCTZ caused a reduced vasorelaxant effect, suggesting that HCTZ has direct vascular relaxant effects via opening of the KCA channel . Clinically, when infused in the brachial artery, HCTZ caused a local vasodilation in hypertensive subjects. The addition channel, of tetraethylammonium, another inhibitor of the KCA chann
e addition channel, of tetraethylammonium, another inhibitor of the KCA chann
in the abolished this effect, further implicating the KCA mechanism of dilation . It is important to note that the thiazide doses used in these infusions resulted in plasma concentrations of 11 μg/ml, which is approximately 10–20 times the plasma concentration found in patients clinically treated with thiazides. Moreover, these findings were contrary to previous studies, which did not find any vasoactivity in the human forearm, although such studies used doses more comparable to those found in clinical practice . Last, the difference in thiazide-induced vasodilation did not appear to vary between hypertensives and normotensives . Because thiazides generally have a negligible blood pressure-lowering effect in normotensives, the clinical impact of this vasodilation is debatable.
Note
the optimal antihypertensive effect with the smallest occurrence of side effects, including alterations in glucose and lipid profiles and hypokalemia. Moreover, because thiazide diuretics can increase the incidence of new-onset diabetes, especially when combined with β blockers, caution is advised in using these drugs above all in patients who are at high risk for developing diabetes, in whom thiazide diuretics should be used at the lowest active dose and possibly in combination with drugs that block the renin-angiotensin system. Finally, the current debate on whether thiazide diuretics are the first-choice drug for most patients with uncomplicated hypertension
Choice of Optimal Dose
dosage choice of thiazide diuretics can be evaluated considering both the effect of different diuretic therapy doses on CV mortality and morbidity and the dose-response curve in terms of antihypertensive efficacy and metabolic effects. With regard to the first issue, a meta-analysis of clinical studies indicated that low-dose (12.5 to 25 mg/d chlorthalidone or hydrochlorothiazide) and high-dose (50 mg/d or more of both drugs) diuretic therapy lowered BP to a similar degree and exerted a similar benefit in reducing stroke, congestive HF, CV, and total mortality, but only low-dose diuretic therapy significantly reduced CHD incidence . The concept of a high dose of diuretic therapy should be replaced by that of optimal dose, which derives from studies that evaluated the dose-response curve of thiazide diuretics, as recently reviewed (
.Data obtained with chlorthalidone so far indicate that the plateau of the dose-response antihypertensive curve is reached with a daily dose of 25 mg and that increasing the dosage does not increase the BP-lowering effect but further increases the occurrence of negative metabolic effects, principally hypokalemia . Data obtained with hydrochlorothiazide, the most widely used thiazide diuretic in clinical practice, are less certain and suggest that the plateau of the dose-response BP-lowering curve is reached with the dose of 50 mg/d, whereas negative metabolic effects, principally hypokalemia, occur in a dose-dependent manner with a dose up to 100 mg/d . Therefore it seems rational to recommend that diuretic treatment with chlorthalidone should start with 6.25 mg in the elderly and 12.5 mg in younger patients, with a maximum dose of 25 mg/d, whereas the starting doses of hydrochlorothiazide should be 12.5 mg in the elderly and 25 mg in younger patients, with a maximum dose of 50 mg/d . Moreover the decision to increase the dosage of these drugs to achieve BP control should be weighed against the occurrence of negative metabolic effect
.Negative Metabolicffects of Thiazide Diuretics
Metabolic effects that can have a negative impact on CV risk profile include alterations in lipids, hypokalemia, impairment of glucose metabolism, and, therefore, the occurrence of type 2 diabetes (13). Although changes in lipid profile, characterized by an increase in total and LDL cholesterol and decrease in HDL cholesterol, have been reported to be modest and transient (13), data from ALLHAT indicate that the increase in total cholesterol is detectable up to 4 yr of follow-up.
Hypokalemia, which as already stated is a dose-dependent phenomenon, can worsen the patient’s prognosis because it can increase the risk for sudden death and CV events among diuretic-treated hypertensive patients and could abolish the benefit of treatment in elderly patients with isolated systolic hypertension (16). Furthermore, hypokalemia can impair glucose metabolism by reducing insulin secretion and insulin sensitivity .
Data from controlled clinical trials, recently reviewed , indicate that the incidence of new-onset diabetes (NOD) is lower in patients who are treated with drugs that block the renin-angiotensin system (RAS), such as ACE inhibitors and angiotensin receptor blockers, as compared with those who are treated with conventional therapy, i.e., thiazide diuretics alone and above all combined with β blockers.
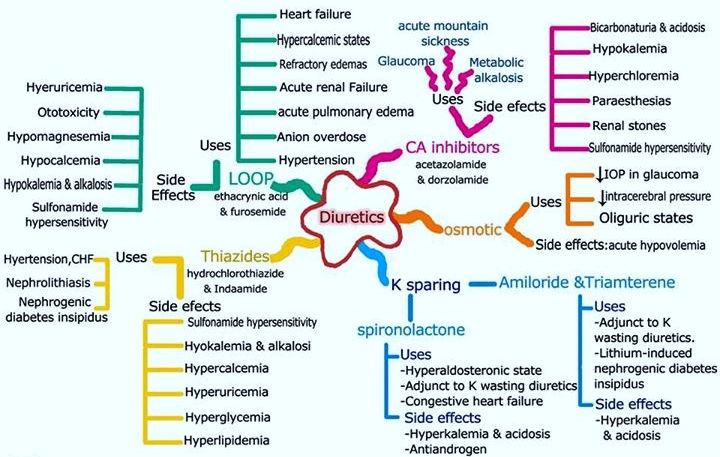
loop diuretics
Loop diuretics are commonly used in the treatment of heart failure. In this condition, fluid accumulates in your body, due to the heart not pumping blood around the body as well as it normally would. So, you may become breathless (as fluid accumulates in the lungs) and your ankles and legs may swell with extra fluid in the tissues (oedema)
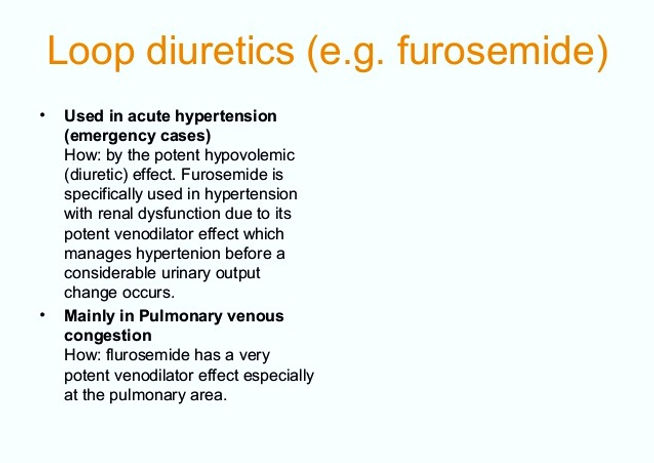
Loop diuretics are also used to treat other conditions which cause fluid to build-up in the body, such as certain liver and kidney disorders. They are also sometimes used to treat high blood pressure (hypertension). (But, a different type of diuretic called a thiazide diuretic is more commonly used to treat high blood pressure.)
They work by making the kidneys pass out more fluid. They do this by interfering with the transport of salt and water across certain cells in the kidneys. (These cells are in a structure called the loop of Henle - hence the name loop diuretic. You have thousands of these loops in each kidney.) As more fluid is passed out by the kidneys, less fluid remains in the bloodstream. So any fluid which has accumulated in the tissues of the lungs or body is drawn back into the bloodstream to replace the fluid passed out by the kidneys. This eases symptoms such as oedema and breathlessness caused y the congestion of fluid.

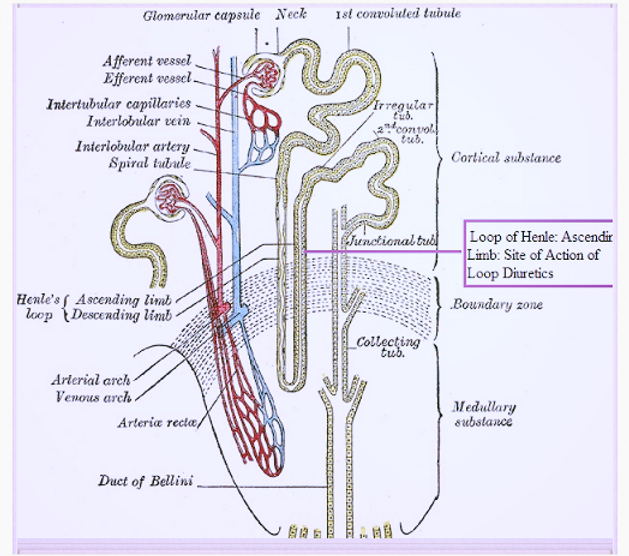
Loop diuretics act in the thick ascending limb of the loop of Henle
Loop diuretics inhibit the NKCC2 (the luminal Na/K/2Cl co-transporter) in the thick ascending limb of the loop of Henle. Urinary output is usually dose dependent and related to the magnitude of fluid accumulation. Water and electrolyte excretion may be increased several times over that observed with thiazide diuretics, since loop diuretics inhibit reabsorption of a much greater proportion of filtered sodium than most other diuretic agents. Therefore, loop diuretics are effective in many patients who have significant degrees of renal insufficiency.
NOTE
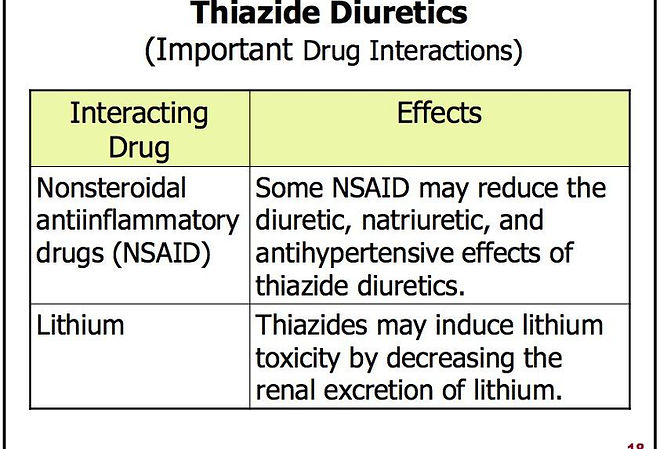
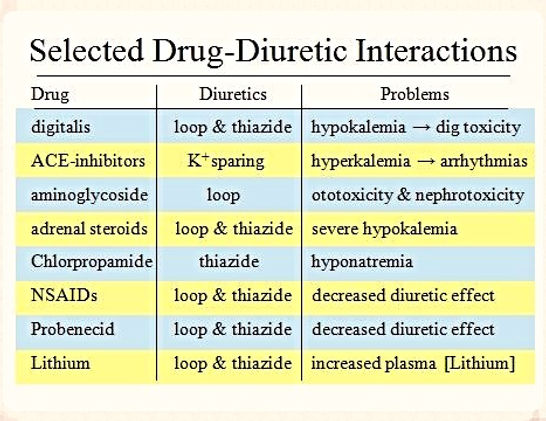
The effects of loop diuretics, thiazides & K-sparing diuretics depend on renal prostaglandin production & can be inhibited by NSAIDs
Loop diuretics produce a more potent diuresis & less vasodilation than thiazide diuretics. Therefore they are less effective in lowering BP than thiazide diuretics
Loop diuretics are drugs of choice for patients with severe edema where a potent diuresis is needed, including:
-
congestive heart failure, cirrhosis of the liver, and renal disease (e.g. GFR <30 ml/min), including the nephrotic syndrome.
-
Short-term management of ascites due to malignancy, idiopathic edema, and lymphedema.
-
Short-term management of hospitalized pediatric patients, other than infants, with congenital heart disease or the nephrotic syndrome.
-
by reducing the lumen voltage gradient that drives cation reabsorption in the loop, loop diuretics increase the excretion of divalent cations (Ca & Mg) & this can be useful in treating disorders causing hypercalcemia (Note: this is an effect opposite from that caused by thiazide diuretics)
-
where a rapid onset of diuresis is desired, e.g., in acute pulmonary edema, or when gastrointestinal absorption is impaired or oral medication is not practicabl



Onset of action is relatively rapid (e.g. usually within 30 minutes after an oral dose of ethacrynic acid or within 5 minutes after an intravenous injection of ethacrynic acid). After oral use, diuresis peaks in about 2 hours and lasts about 6 to 8 hours. Most loop diuretics have a short duration of action & require twice-daily dosing.




Contraindications: anuria. If increasing electrolyte imbalance, azotemia, and/ or oliguria occur during treatment of severe, progressive renal disease, the diuretic should be discontinued.
Furosemide (fure oh' se mide) was the first loop diuretic to be approved in the United States (1966) and is still widely used with more than 37 million prescriptions filled yearly. Furosemide is available in tablets of 20, 40 and 80 mg in generic forms and under the brand name Lasix. Furosemide is also available as an oral solution and as a liquid solution for injection. The usual adult dose of furosemide is 20 to 320 mg daily, given in one to three divided doses.
Ethacrynic (eth a krin' ik) acid was the second loop diuretic to be approved for use in the United States (1967), but is now rarely used; it remains available in 25 mg tablets and a solution for intravenous use generically and under the brand name Edecrin. The usual oral adult dose is 25 to 100 mg in one to three divided doses daily.is a medication used to treat fluid build-up due to heart failure, liver scarring, orkidney disease. It may also be used for the treatment of high blood pressure. The amount of medication required depends on the person in question. It can be taken intravenously or by mouth. When taken by mouth it typically begins working within an hour while intravenously it typically begins working within five minutes.
side effects
low blood pressure with standing,
ringing in the ears,
sensitivity to the sun.
Potentially serious side effects includeelectrolyte abnormalities
, low blood pressure,
hearing loss.
Bumetanide (bue met' a nide) is a potent loop diuretic that was approved for use in the United States in 1983 and continues to be used for the treatment of edema. Bumetanide is available as tablets of 0.5, 1 and 2 mg in generic forms and under the trade name of Bumex. The usual oral adult dose is 0.5 to 2 mg in two or three divided doses daily.
is a loop diuretic of the sulfamyl category to treatheart failure. It is often used in people in whom high doses of furosemide are ineffective. It is marketed by Hoffmann-La Roche. The main difference between the two substances is inbioavailability and pharmacodynamic potency. Furosemide is incompletely absorbed in the intestine (60%), and there are substantial inter- and intraindividual differences in bioavailability (range 10-90%). Bumetanide is almost completely absorbed (80%), and the absorption is not altered when it is taken with food. It is said to be a more predictable diuretic, meaning that the predictable absorption is reflected in a more predictable effect.
Bumetanide is 40 times more potent than furosemide for patients with normal renal function.
In the brain, bumetanide blocks the NKCC1cation-chloride co-transporter, and thus decreases internal chloride concentration inneurons. In turn, this concentration change makes the action of GABA more hyperpolarizing, which may be useful for treatment of neonatalseizures, that quite often are not responsive to traditional GABA-targeted treatment, such asbarbiturates. Bumetanide is therefore currently under evaluation as a prospective antiepileptic drug.
side effects
-
Abdominal pain
-
blurred vision
-
confusion
-
decreased urine output
-
dizziness
-
drowsiness
-
dry mouth
-
fast or irregular heartbeat
-
flushed, dry skin
-
fruit-like breath odor
-
headache
-
increased hunger
-
increased thirst
-
increased urination
-
irritability
-
joint pain, stiffness, or swelling
-
loss of appetite
-
Rare
-
Agitation
-
back pain
-
black, tarry stools
-
bleeding gums
-
blood in the urine or stools
-
chest pain
-
convulsions (seizures)
-
deep or fast breathing with dizziness
-
dizziness, faintness, or lightheadedness when getting up from a lying or sitting position suddenly
-
fever
-
hallucinations
-
hives
-
increase in heart rate
-
increased blood pressure
-
numbness of feet, hands, and around the mouth
-
pinpoint red spots on the skin
-
rapid breathing
-
stiff neck
-
sunken eyes
-
trembling, jerking of hands
-
unusual bleeding or bruising
-
weight gain
-
wrinkled skin
-
If any of the following symptoms of overdose occur while taking bumetanide, get emergency help immediately:
Symptoms of overdose
-
Difficulty breathing
-
pain in chest, groin, or legs, especially the calves
-
severe, sudden headache
-
slurred speech
-
sudden loss of coordination
-
sudden, severe weakness or numbness in the arm or leg
-
sudden, unexplained shortness of breath
-
unusual drowsiness, dullness, or feeling of sluggishness
-
vision changes
-
care professSome of the side effects that can occur with bumetanide may not need medical attention. As your body adjusts to the medicine during treatment these side effects may go away. Your health care professional may also be able to tell you about ways to reduce or prevent some of these side effects. If any of the following side effects continue,
Rare
-
Decreased interest in sexual intercourse
-
diarrhea
-
difficulty with moving
-
ear discomfort
-
feeling of constant movement of self or surroundings
-
inability to have or keep an erection
-
itching skin
-
loss in sexual ability, desire, drive, or performance
-
muscle or bone pain
-
muscle stiffness
-
nipple tenderness
-
pain, swelling, or redness in joints
-
rash
-
sensation of spinning
-
shorter than usual time to ejaculation of semen
-
trouble with hearing
-
upset stomach
-
-
loss of mental alertness
-
lower back, side, or stomach pain
-
mood or mental changes
-
muscle pain or cramps
-
nausea or vomiting
-
numbness or tingling in the hands, feet, or lips
-
shortness of breath
-
sweating
-
swelling of face, hands, feet, ankles, or lower legs
-
troubled breathing
-
unexplained weight loss
-
unusual tiredness or weakness
-
weak pulse
Torsemide( examide) was approved for use in edema in the United States in 1993 and is still in common use used for both edema and hypertension. Torsemide (tor' se mide) is available in tablets of 5, 10, 20 and 100 mg in generic forms and under the brand name of Demadex. Solutions are available for intravenous use as well. The usual oral adult dose is 5 to 100 mg daily in one or two divided doses.
Compared with other loop diuretics, torasemide has a more prolonged diuretic effect than equipotent doses of furosemide and relatively decreased potassium loss. No evidence of torasemide-induced ototoxicity has been demonstrated in humans.
-
Chest pain
-
convulsions
-
decreased urination
-
diarrhea
-
dry mouth
-
electrocardiogram (ECG) changes
-
increased thirst
-
irregular heartbeat
-
loss of appetite
-
mood changes
-
muscle pain or cramps
-
nausea or vomiting
-
numbness or tingling in hands, feet, or lips
-
shortness of breath
-
swelling of hands, ankles, feet, or lower legs
-
unusual tiredness or weakness
Rare
-
Black, tarry stools
-
dizziness, faintness, or lightheadedness when getting up from a sitting or lying position suddenly
-
ringing or buzzing in the ears or any hearing loss
-
skin rash
If any of the following symptoms of overdose occur while taking torsemide, get emergency help immediately:
Symptoms of overdose
-
Blurred vision
-
coma
-
confusion
-
decreased urine output
-
dizziness
-
drowsiness
-
fainting
-
fast heartbeat
-
fatigue
-
headache
-
increase in heart rate
-
irritability
-
lightheadedness
-
rapid breathing
-
sunken eyes
-
sweating
-
weak pulse
-
wrinkled skin
Some torsemide side effects may not need any medical attention. As your body gets used to the medicine these side effects may disappear
Use of the loop diuretics has not been associated with an increased rate of serum aminotransferase elevations. There have been only rare reported cases of clinically apparent liver injury associated with loop diuretics and most of these reports have not been very convincing. Interestingly, furosemide causes a direct hepatotoxicity in mice and has been used as an animal model of drug induced liver injury. This injury does not appear to occur in humans. Thus, clinically apparent liver injury from the loop diuretics must be exceeding rare, if it occurs at all.
Mechanism of Injury
The cause of the rare occurrence of clinically apparent liver injury associated with the loop diuretics is not known. These agents are metabolized minimally by the liver and generally have rapid renal excretion.
Outcome and Management Cases of clinically apparent liver injury due to the loop diuretics have been too few to characterize their severity and course. There have been no published instances of acute liver failure or chronic liver injury attributed to any of the loop diuretics. Cross reactivity among the four agents is unlikely because of the variability of their chemical structure.